zinc stearate reaction
The safest products score well by both measures with a low hazard rating and. Toluene helped zinc stearate to turn into the corresponding methyl esters by making zinc stearate partly soluble in a raised temperature as well as making the reaction product soluble.
ZINC stearate C36H70O4Zn CID 11178 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more.

. Eyes skin respiratory system NIOSH 2022. National Institute for Occupational Safety and Health NIOSH Recommended Exposure Level TO ZINC STEARATE total dust-air. Metallic Stearates Control Over Discoloration Metallic Stearates can only control color of a polyolefin matrix if the system is in balance.
Raising temperature from room temperature RT to 45C or 90C allowed the reaction to proceed faster. It is a white powder and is insoluble in water. 1214 In the previous section ArH.
Most zinc compounds are not toxic and dont show any symptoms with acute exposures. A preparation technology of zinc stearate comprises the following steps that 1 glyceryl tristearate and water are hydrolyzed under the action of a catalyst and an antioxidant. 2 zinc oxide and a catalyst are added stepwise condensing dewatering and salt forming are conducted and zinc stearate is synthesized.
Portions of melted stearic are added down the condenser at 15-minute intervals until an additional 240 g. Types of primary antioxidants and levels determine the degree of interaction between metallic stearates. Zinc stearate is high in preparation yield good in color and cluster low.
In this work the simultaneous transesterification and esterification reactions of olive pomace oil with methanol catalyzed by zinc stearate were studied. 10H time-weighted average 5 mgm 3. Inhalation ingestion skin andor eye contact.
In the fusion process an increase in mixing rate decreased the induction time occurring at the beginning of the reaction. Method of producing zinc stearate involves reacting stearic acid and zinc hydroxide with heating and intense stirring followed by heat treatment filtration drying and packaging. 284 g 1 mole total has.
Zinc stearate is produced from various reactions including the reaction of zinc oxide and stearic acid which is shown in the following. Soluble zinc salts of fatty acid such as zinc 2-ethyIhexanoate are also used. It is widely used as a release agent for the production of many kinds of objects.
Evolved hydrochloric acid reacts with zinc stearate to produce zinc chloride according to the following equation C17H35COO2Zn2HCI 2C17H35COOHZnCl2 Also zinc stearate reacts with PVC defect sites to produce zinc chloride. Although the excess alcohol could not react with the resulting zinc phosphonic acid salt it could force the newly formed zinc stearate gradually but completely back onto the existing nanocrystals. Excerpt from NIOSH Pocket Guide for Zinc stearate.
Zinc Stearate is a material that is easily soluble in water ultra-fine and with good dispersion compatibility. After the reaction has proceeded for 1 hour 10-g. Zinc stearate was synthesized by precipitation method through two steps.
The formation of mesh network is determined to occur slightly earlier than that of the network domains using a combination of in situ zinc K-edge XAFS and DSC measurements. Mainly used as lubricant and release agent for styrene resin phenolic resin and amino resin. Addition of zinc stearate is known to be technically a superior acid scavenger since it helps to avoid early discoloration of a large series polymer formulations but it holds for being physically irritant in humans.
Respiratory irritation and bronchospasm may develop after inhalation. Although there is no specific information available on the concentration of the exposure. In residues that had been held at 200 oC for 30 min prior to quenching distinct zinc stearate peaks were present.
The Skin Deep scoring system was designed to help the public understand whether a product is safe to use or whether it contains ingredients of concern. Particularly in the presence of stearic acid and zinc stearate. At the same time it also has the function of vulcanization activator and softener in rubber.
National Institute for Occupational Safety and Health NIOSH Recommended Exposure Level TO ZINC STEARATE respirable fraction-air. Irritation eyes skin upper respiratory system. These applications exploit its non-stick properties.
Based on animal studies with rat and mouse models high doses of zinc stearate affect the lungs and thorax. In cosmetics zinc stearate is a lubricant and thickening agent used to improve texture. Upon the addition of a small amount of stearic acid or phosphonic acid immediate partial dissolution of ZnO nanocrystals took place.
10H time-weighted average 10 mgm 3. Rubber polyurethane polyester processing system powder metallurgy. Conveying liquid stearic acid by a pump allowing the liquid stearic acid to go through a flow meter for metering and then enter a zinc stearate.
12 This is probably due to the acceleration of sulfur cross-linking of the mesh network by the dinuclear bridging bidentate zincstearate complex intermediate. This catalyst is a crystalline solid at room temperature but it is soluble in the reaction medium at reaction temperature. Neutralization of stearic acid by sodium hydroxide then double decomposition using zinc sulphate to precipitate zinc.
While the melting point of the zinc stearate prepared by the precipitation process was found to be about 122C by optical microscopy it was slightly lower than 122C for zinc stearate produced by the fusion process. However moderate toxicity can result in nausea vomiting abdominal pain and diarrhea. This chemical is an example of metal soaps and is insoluble in water and polar solvents such as alcohol.
In samples formed from zinc oxide and the ester zinc stearate was detected similarly after reaction at 200 oC for 30 min. The influence of a double bond in the middle of an otherwise flexible hydrocarbon chain on the melting of such assemblies has been investigated by comparing the melting behavior of zinc stearate and zinc oleate. The technology comprises the following steps.
Zinc stearate has different ratios of palmitic and stearic acids. 54 Exposure to zinc stearate over a long time may develop extensive fibrosis. 367 The solid residue quenched from 600 after reaction of ethyl stearate with zinc powder gave X-ray diffraction patterns for both zinc.
It is an activator for accelerated rubber sulfur vulcanization. Zinc stearate is an organic substance with a chemical formula of C36H70O4Zn. As discovered in the early days of v.
Considering the molar masses of each chemical substance in the reaction gmol -1 Zn 814 gmol -1 stearic acid 2845 gmol -1 and water 18 gmol -1 and the respective stoichiometric. Every product and ingredient in Skin Deep gets a two-part score one for hazard and one for data availability. Exceeding the buffer balance of pH and free acidity towards the basic side will increase color.
Zinc stearate has surfactant properties that cause the formation of an emulsion in the reaction system. It is valued by manufacturers because of the delicate feeling it gives to the finished products good heat resistance excellent transparency yellowing resistance quick-drying and increased sandability. By monitoring features in the infrared spectra that are characteristic of the global conformation of the hydrocarbon chain it is shown that the double.
When reacting stearic acid and zinc hydroxide hydrochloric acid is further added to the mixture as a catalyst.
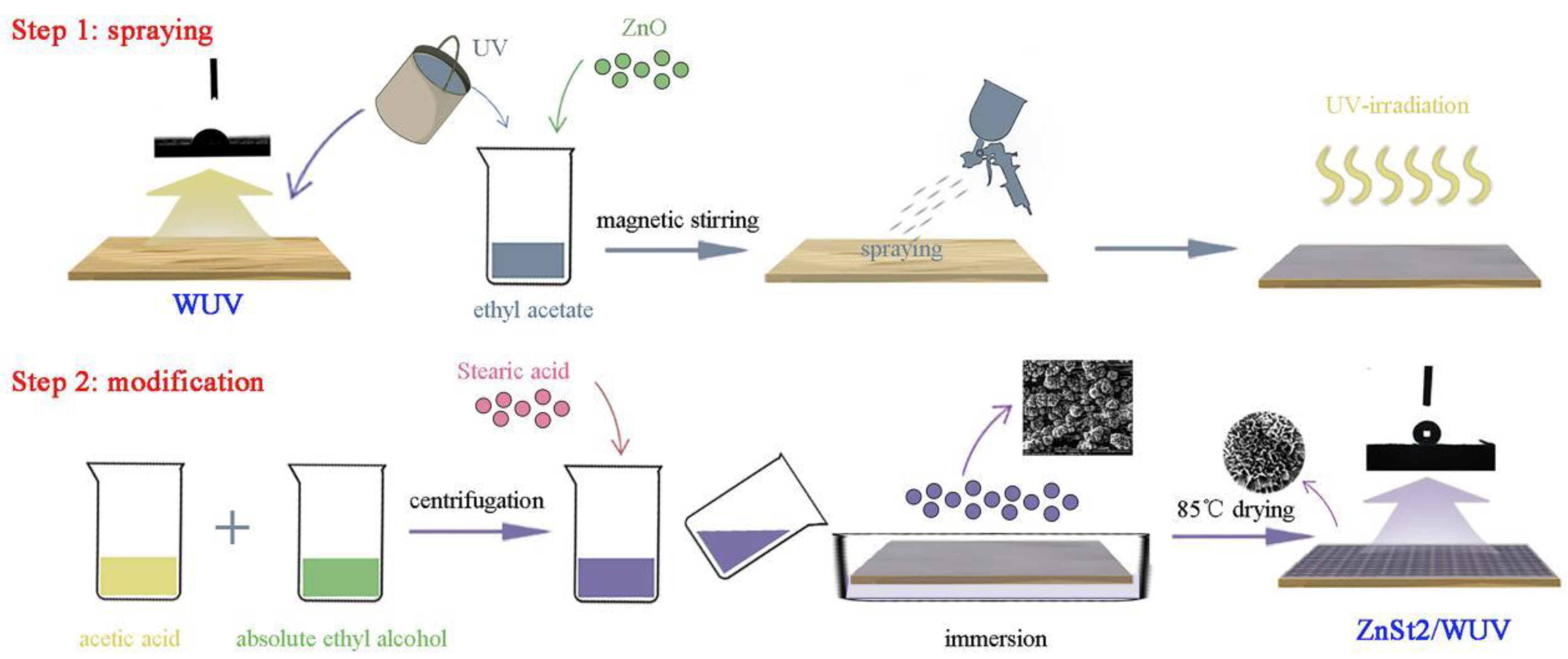
Polymers Free Full Text Preparation And Characterization Of Waterborne Uv Lacquer Product Modified By Zinc Oxide With Flower Shape Html

Influence Of Two Different Alcohols In The Esterification Of Fatty Acids Over Layered Zinc Stearate Palmitate Sciencedirect
Comparison Of Commercial Zinc Stearate Reference Sample In Atr Mode And Download Scientific Diagram

Zinc Stearate Meets Usp Testing Specifications Sigma Aldrich
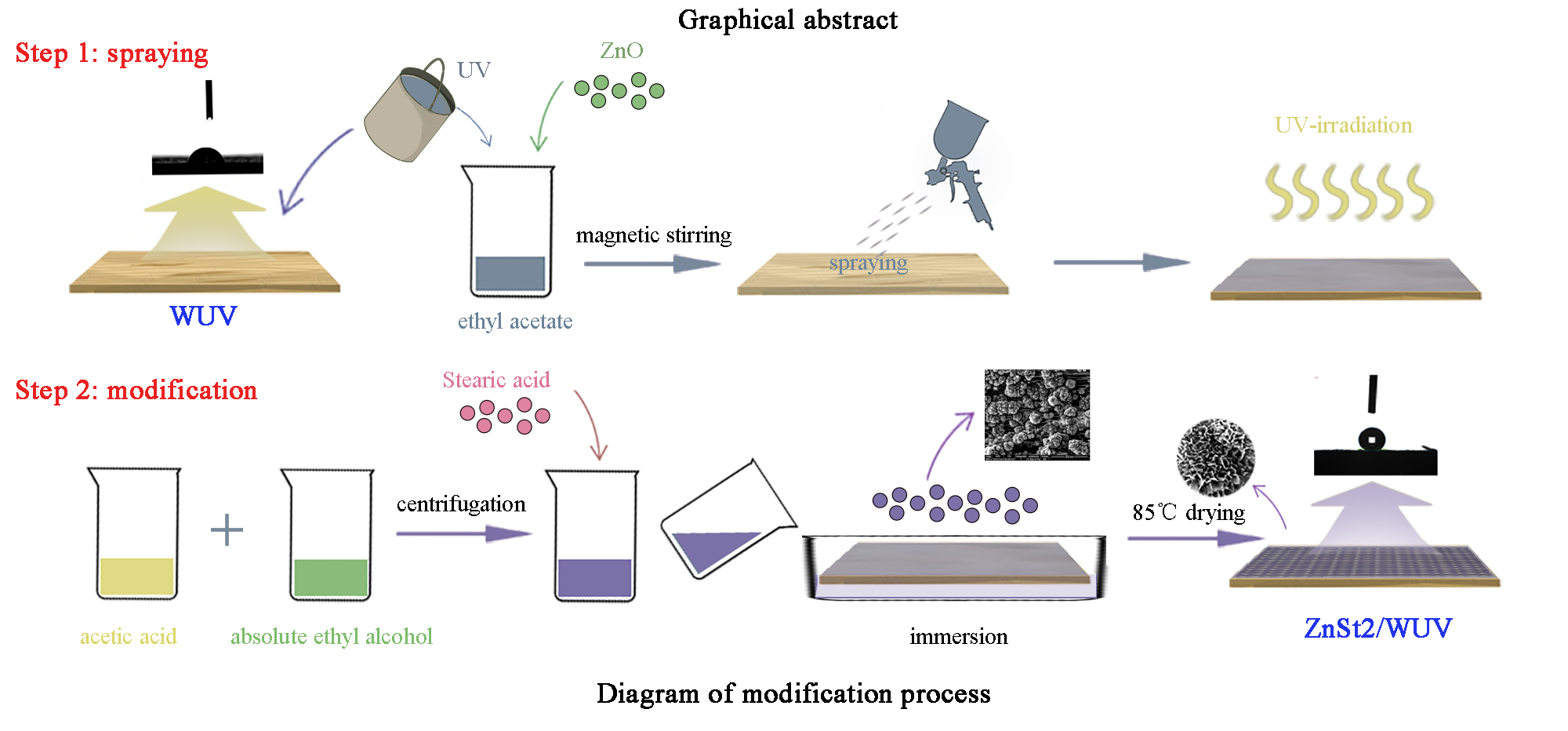
Polymers Free Full Text Preparation And Characterization Of Waterborne Uv Lacquer Product Modified By Zinc Oxide With Flower Shape Html

Reaction Between Zno And Stearic Acid Forming Zinc Stearate And The Download Scientific Diagram

Hascovir Control Max 0 4g X 30 Tablets Uk Headache And Dizziness Cold Sore Atopic Skin
Stearic Acid Sodium Salt Fatty Acids And Derivatives Oils And Greases Natural Reference Materials Chemicals Carl Roth International

Measured Characteristics Of The Prepared Zinc Stearate Download Table

Ofra Glowgoals Highlighter Highlighter Cosmetics Laboratory Shimmer
Zinc Stearate C36h70o4zn Pubchem

Effect Of Synthesized Zinc Stearate On The Properties Of Natural Rubber Vulcanizates In The Absence And Presence Of Some Fillers Sciencedirect

Zinc Stearate Formation And Vulcanization Mechanisms For Step 1 Download Scientific Diagram
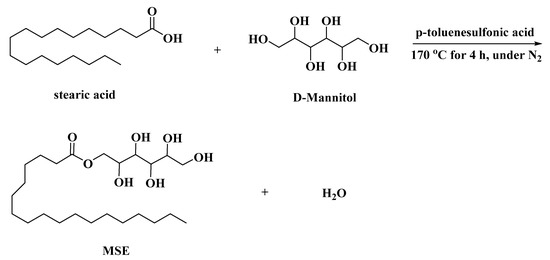
Polymers Free Full Text Design And Synthesis Of A New Mannitol Stearate Ester Based Aluminum Alkoxide As A Novel Tri Functional Additive For Poly Vinyl Chloride And Its Synergistic Effect With Zinc Stearate
Reaction Between Zno And Stearic Acid Forming Zinc Stearate And The Download Scientific Diagram

Reaction Between Zno And Stearic Acid Forming Zinc Stearate And The Download Scientific Diagram

The Chemistry Of Limescale Teaching Chemistry Chemistry Education Chemistry



Comments
Post a Comment